International Clinical Trials Day: how our experts are improving women’s health through inclusion
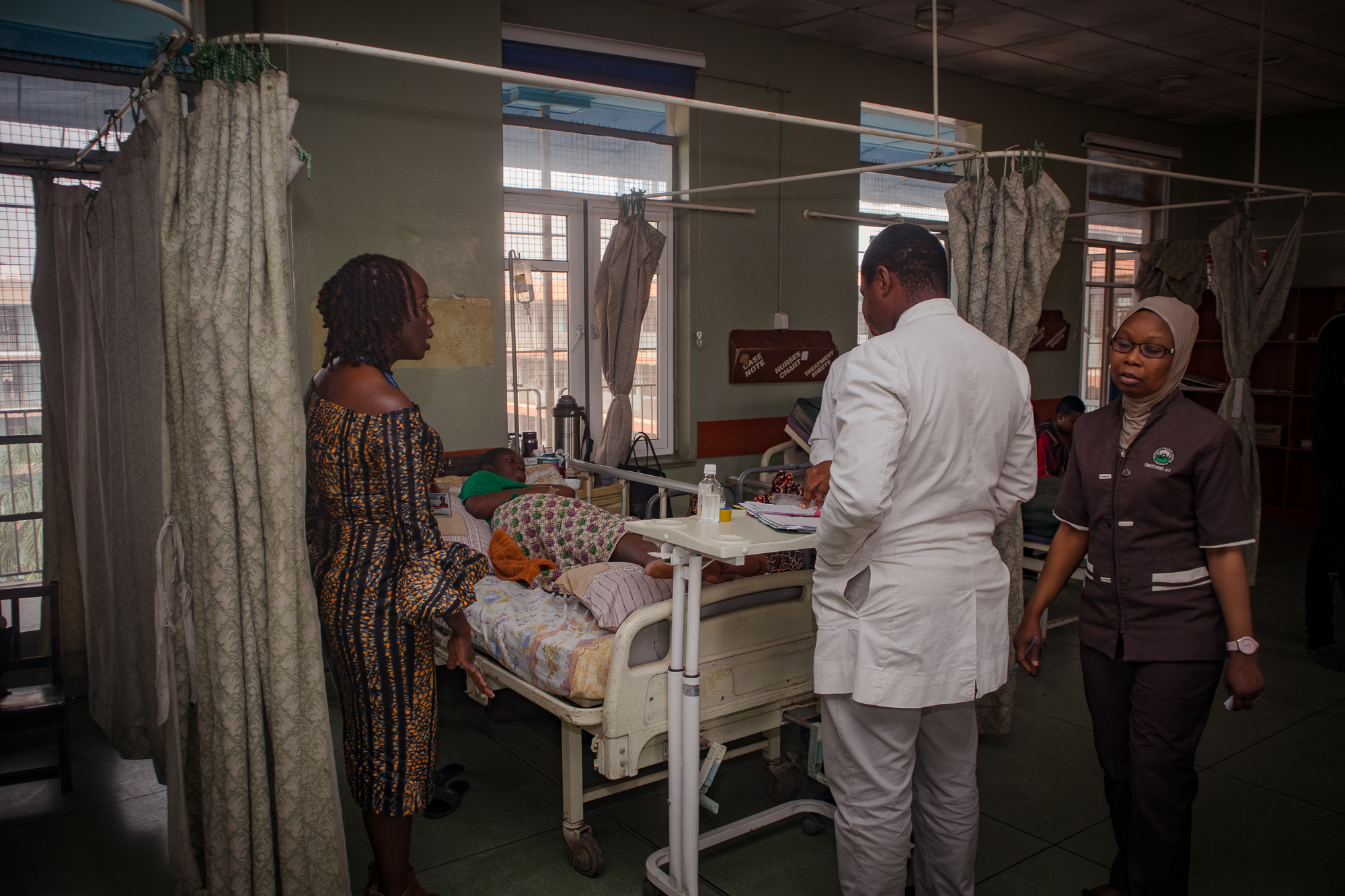
Credit: LSHTM. Professor Nike Bello, WOMAN Trials National Coordinator for Nigeria, examines a patient at University College Hospital Ibadan.
Involve more pregnant women in clinical research
In a recent comment in The Guardian, Principal Investigator of the I’M WOMAN Trial, Dr Amy Brenner shared how the absence of pregnant and breastfeeding women in clinical trials means many women don’t receive treatments which could benefit their health.
She added that mothers may be apprehensive about the safety of clinical trials or reluctant to take part out of concern for their babies, but that reducing health inequities would ultimately lead to more interventions for women’s health.

Dr Brenner said: “It is particularly concerning that there are more male-only trials than female-only trials as, while they may be disease-specific, it is certainly not true that there are more male-only than female-only diseases.”
Find out more
Pregnant (and breastfeeding) women have been systematically denied the benefits of new knowledge by being excluded from clinical trials. Historically, the main concerns have been that medicines might harm the unborn baby and changes in the woman’s body during pregnancy might affect the way the treatment works – even though most women take some form of medication during pregnancy.
Professor Ian Roberts, Co-lead of the WOMAN-2 Trial, discussed how including pregnant women in clinical trials is needed to learn whether treatments are effective.
He said: “As a result of this exclusion, there is a yawning knowledge gap on the effectiveness and safety of medicines in pregnancy, and this causes major problems when pregnant women need healthcare.

“Researchers should collaborate with pregnant women to ensure their participation. Pregnant women don’t need paternalistic protection from participating in research – they need results that can inform their care and lead to better outcomes.
“There are, of course, special considerations in pregnancy. Changes in drug absorption, distribution and metabolism may require a different dose, but exclusion is not the answer.”
Find out more
Recognise how patient care improves clinical research
The I’M WOMAN Trial is working to address some of the barriers women face when accessing a clinical trial by involving clinical research nurses and midwives. Their direct contact with patients allows them to better understand and address women’s health concerns, and perhaps even improve the recruitment of women to clinical trials.
Nurses and midwives are part of the trial teams that administer tranexamic acid – a drug used to treat excessive bleeding after childbirth – and reassure patients who are taking part in the clinical trial that it is a safe and effective drug.

Sumaira Shaheen Abbasi is a nurse at Jinnah Postgraduate Medical Center, Pakistan. She works in the I’M WOMAN Trial team to assess whether giving intramuscular tranexamic acid is as effective as giving it intravenously.
She said: “In Pakistan, patients mostly have no opportunity to be involved in a clinical trial. However, in this hospital, we can give clinical trial care to help the mother and babies by reducing their risk severe postpartum bleeding.
“When I give tranexamic acid intravenously or intramuscularly, I can see that patients are really comforted by having answers about postpartum haemorrhage (PPH). Their babies are born very strong and after they are stable, they are properly discharged. Then, if they have any problem in the postpartum period they can come to the hospital.
“I tell the doctor if there is any kind of abnormality in the patient or discomfort immediately. If I tell the doctor the patient is bleeding, we can treat them accordingly.”
Clinical trials should address inequity in women’s health
Professor Haleema Shakur-Still, Co-lead or of the WOMAN-2 Trial, discussed the importance of lifesaving interventions, including TXA, following her experience as a nurse where she witnessed a woman die from PPH.
“I was working on the CRASH clinical trial at the time testing tranexamic acid on traumatic bleeding injury. A woman arrived having given birth outside the hospital. She came in extremely unwell, and we could hear resuscitation behind the curtains, then there was this wailing, and we learned this woman had died from PPH.
“When I spoke to the doctor, they said they needed more treatments for women with PPH and asked why women in this situation were not being tested.
“We know from the WOMAN Trials; tranexamic acid reduces the risk of a woman bleeding to death by about a third. I believe the WOMAN Trials have made a difference to women worldwide.”

Professor Haleema Shakur-Still and Dr Amy Brenner at Amana Regional Referral Hospital, Tanzania.
Include women, even in “male-dominated” areas of health
Richard Evans, a Senior Manager at LSHTM’s Clinical Trials Unit the CTU is looking at ways of including more women in cardiovascular trials, which have long been dominated by male patients.
Cardiovascular disease has largely and mistakenly been understood as a predominately male disease, meaning that most trials on the issue are not designed with women in mind.
He said: “There are unanswered questions on women’s cardiovascular health that aren’t being considered as subjects for clinical trials because of a lack of confidence that these trials can be delivered.”
“Because there is an idea that it’s challenging to recruit women to cardiovascular trials, there is sometimes a reluctance to fund research in women. This clearly needs to change in order to address this issue.”

To understand effective treatments, researchers involve patients, carers and the public in designing and running trials – known as patient and public involvement (PPI). As this can improve gender and ethnic diversity, as can the numbers of women speaking about their experiences being involved with clinical trials.
The team has a trial starting soon where their plans for greater inclusion will be put into practice. The trial will have enhanced PPI and specific monitoring to look at diversity (sex and ethnicity) in real time.
The trial is called REPRIEVED and focuses on people with Heart Failure with preserved Ejection Fraction (HFpEF). This is a condition caused by a stiffening of the heart muscle, which occurs more often in women than in men. REPRIEVED will look at the role played by stenting in improving the way the heart functions.

